For further information about trans-2-aminocyclohexanol based pH-triggered molecules, it is better start with simple molecule with key functional groups, then extend it to full variety of all derivatives.
The structure of unprotonated form of trans-2-aminocyclohexanol backbone(cis) is as follows:
the distance between the hydroxonium and trans-2-aminocyclohexanol backbone is about 12 A. the energy is -443.2855 a.u.
The structure of protonated form of trans-2-aminocyclohexanol backbone(cis) is as follows:
The energy is -443.4171 a.u. ,
The energy is -443.2927 a.u.
unprotonated & unhydrate protonated & unhydrate
-443.2855 a.u. -443.2927 a.u.
protonated & hydrate
-443.4171 a.u.
the energy difference is 0.1316 a.u. x 627.5095kcal/mol= -82.58 kcal/mol
Even this is total energy difference, not free energy, it still tells us how stable the molecule is when it is protonated.
(if 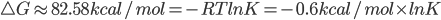 ---->K will be a huge number.
---->K will be a huge number.
Let's look what's the energy difference of trans-2-aminocyclohexanol backbone(trans).
this is the situation with two water molecules.
........
transition from trans to cis:





